The Impact of Nanotechnology on Drug Delivery Systems
Nanotechnology, the manipulation of matter at the nanoscale (typically 1 to 100 nanometers), has emerged as one of the most transformative fields in modern science. In particular, nanotechnology has had a profound impact on drug delivery systems (DDS), offering new solutions to long-standing challenges in medicine. These advancements have the potential to revolutionize the treatment of a wide range of diseases, from cancer to genetic disorders. This article explores how nanotechnology is reshaping drug delivery, discussing its benefits, challenges, and examples of its applications.
Introduction to Nanotechnology in Drug Delivery

The delivery of therapeutic agents has always been a challenge in medicine. Traditional drug delivery methods, such as oral and intravenous administration, often face significant barriers in ensuring that drugs reach their intended site of action in the body. These conventional systems can result in poor bioavailability, systemic toxicity, and insufficient therapeutic efficacy.
Nanotechnology offers a solution to many of these problems by enabling the creation of drug delivery systems at a molecular or nanoscale level. Nanoparticles, which are particles that range in size from 1 to 100 nanometers, are used to deliver drugs more effectively by improving their solubility, stability, and targeting capabilities. The primary goal is to achieve more effective treatments with fewer side effects by delivering drugs directly to the affected cells or tissues.
Nanocarriers: Types and Applications in Drug Delivery
Nanocarriers are engineered vehicles designed to carry therapeutic agents to specific targets in the body. These carriers can be made from a variety of materials, including lipids, polymers, metals, and ceramics, each offering distinct advantages in drug delivery applications.
1. Liposomes:
Liposomes are spherical vesicles composed of a lipid bilayer that can encapsulate both hydrophilic and hydrophobic drugs. They are among the earliest and most studied nanocarriers in drug delivery. Liposomes are beneficial for improving the pharmacokinetics and biodistribution of drugs, and they can reduce toxicity by targeting drugs to specific areas.
Example: Doxil, a liposomal formulation of the chemotherapy drug doxorubicin, has been used to treat various cancers. The encapsulation of the drug in liposomes reduces its side effects and increases its accumulation in tumor tissues, leveraging the enhanced permeability and retention (EPR) effect.
2. Polymeric Nanoparticles:
Polymeric nanoparticles are made from biodegradable and biocompatible materials such as poly(lactic-co-glycolic acid) (PLGA). These nanoparticles can be engineered to release drugs in a controlled manner, making them suitable for chronic disease management and targeted drug delivery.
Example: The anticancer drug Paclitaxel is often delivered through polymeric nanoparticles, which enable slow and sustained release over time, enhancing the drug’s effectiveness and reducing the need for frequent administration.
3. Nanostructured Lipid Carriers (NLCs):
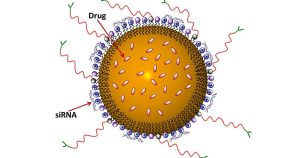
NLCs combine the benefits of both liposomes and solid lipid nanoparticles, offering improved stability and the ability to encapsulate a wider range of drugs. NLCs are also suitable for controlled release and have potential applications in both oral and parenteral drug delivery.
Targeted Drug Delivery: Active and Passive Targeting with Nanotechnology
A key advantage of nanotechnology in drug delivery is its ability to improve the specificity and precision of drug delivery, minimizing systemic side effects. There are two main types of targeting strategies used in nanotechnology-based drug delivery: passive and active targeting.
1. Passive Targeting:
Passive targeting relies on the unique characteristics of certain tissues or cells, such as tumors, to facilitate drug delivery. The Enhanced Permeability and Retention (EPR) effect is a phenomenon where nanoparticles accumulate in tumor tissues due to their leaky blood vessels. This passive accumulation allows for high concentrations of the drug in the tumor, while minimizing exposure to healthy tissues.
Example: Nanoparticles such as Doxil (liposomal doxorubicin) exploit the EPR effect to preferentially deliver the drug to cancer cells, enhancing therapeutic outcomes and reducing toxic side effects commonly associated with conventional chemotherapy.
2. Active Targeting:
Active targeting involves the modification of nanoparticles with specific ligands (e.g., antibodies, peptides) that recognize and bind to receptors overexpressed on the surface of target cells. This strategy provides a high degree of specificity, allowing drugs to be delivered directly to diseased tissues or cells, such as cancer cells.
Example: HER2-targeted liposomal nanoparticles have been developed for the treatment of breast cancer. These nanoparticles are conjugated with monoclonal antibodies targeting the HER2 receptor, which is overexpressed in certain types of breast cancer, ensuring that the drug is delivered specifically to cancer cells.
Nanotechnology in Overcoming Drug Solubility and Bioavailability Issues
Many drugs suffer from poor solubility, which limits their absorption and bioavailability. Nanotechnology offers innovative solutions to improve the solubility of poorly water-soluble drugs. Nanocarriers can enhance the solubility and stability of such drugs, making them more effective.
1. Nanocrystals:
Nanocrystals are small particles of a drug in the nanometer range that have significantly increased surface areas. This increased surface area improves the dissolution rate of poorly soluble drugs, leading to better absorption.
Example: The drug Griseofulvin, an antifungal medication, has been formulated as nanocrystals to enhance its solubility and bioavailability. This formulation results in more efficient therapeutic outcomes compared to conventional forms of the drug.
2. Liposomes and Micelles:

Liposomes and micelles are also used to improve the solubility of hydrophobic drugs. These nanocarriers encapsulate hydrophobic drugs in their core, preventing degradation and ensuring that the drug is delivered efficiently to the target site.
Example: The use of micellar formulations of Taxol (paclitaxel) has been shown to improve the bioavailability of the drug, enhancing its effectiveness in cancer treatment.
Controlled and Sustained Drug Release Using Nanotechnology
One of the most significant advantages of nanotechnology in drug delivery is the ability to control and sustain the release of drugs over time. This approach not only enhances the efficacy of drugs but also reduces the frequency of administration, improving patient compliance.
1. Controlled Release:
Controlled release formulations are designed to release a drug at a predetermined rate, allowing for a continuous therapeutic effect over an extended period. This is particularly useful for chronic conditions that require long-term treatment.
Example: Insulin is commonly delivered using polymeric nanoparticles that control its release, mimicking the natural insulin release process in the body.
2. Sustained Release:
Sustained release formulations are designed to release drugs over a prolonged period, maintaining a steady drug concentration in the body. This approach reduces the peak-to-trough fluctuations seen with traditional drug delivery methods.
Example: Depo-Provera, a sustained-release contraceptive formulation, uses nanoparticles to provide a continuous release of the hormone, reducing the need for frequent injections.
Nanotechnology in Gene Delivery and RNA-Based Therapies
Nanotechnology has opened new possibilities in the field of gene therapy and RNA-based treatments. Nanocarriers such as lipid nanoparticles (LNPs) have become essential for the delivery of genetic materials like mRNA, siRNA, and plasmid DNA.
1. Lipid Nanoparticles for mRNA Delivery:
Lipid nanoparticles were instrumental in the development and successful distribution of mRNA-based COVID-19 vaccines (e.g., Pfizer-BioNTech and Moderna). These nanoparticles encapsulate the mRNA, protecting it from degradation and facilitating its delivery into cells where it can trigger an immune response.
2. siRNA and Gene Silencing:
Nanotechnology is also being used to deliver small interfering RNA (siRNA) for gene silencing, providing potential treatments for genetic disorders and cancers. Nanocarriers protect siRNA from enzymatic degradation and ensure its efficient delivery to target cells.
Example: Onpattro, the first FDA-approved RNA interference (RNAi) drug, uses lipid nanoparticles to deliver siRNA for the treatment of hereditary transthyretin amyloidosis.
Theranostics: The Combination of Therapy and Diagnosis through Nanotechnology
Nanotechnology is paving the way for the development of theranostic systems, which combine diagnostic and therapeutic functions into a single platform. These smart nanoparticles can both deliver drugs and provide real-time diagnostic information, enhancing treatment precision and effectiveness.
Example: Theranostic nanoparticles have been developed that can be used for both cancer treatment and imaging. These nanoparticles are engineered to carry chemotherapy drugs and also contain imaging agents, such as fluorescent dyes or MRI contrast agents, enabling doctors to monitor the drug’s distribution in real-time.
Challenges and Safety Concerns in Nanotechnology-Based Drug Delivery
While nanotechnology offers immense potential, there are several challenges and safety concerns that must be addressed before these technologies can be widely adopted in clinical practice.
1. Toxicity and Biocompatibility:
There are concerns about the long-term toxicity of nanoparticles, especially when they accumulate in the body. Researchers are focusing on developing biocompatible and biodegradable nanoparticles that break down into non-toxic substances after they have delivered their payload.
2. Manufacturing Challenges:
The scale-up of nanotechnology-based drug delivery systems presents challenges in terms of consistent quality and reproducibility. Developing standardized protocols for the manufacture of nanoparticles is essential for ensuring safety and efficacy.
Future Prospects of Nanotechnology in Drug Delivery

The future of nanotechnology in drug delivery is promising, with continued advancements in materials science, gene therapy, and theranostics. As regulatory frameworks evolve, the widespread use of nanotechnology in clinical settings will likely become a reality, offering personalized and more effective treatment options for patients.
Conclusion
Nanotechnology has dramatically enhanced the field of drug delivery by improving the targeting, solubility, bioavailability, and release profiles of drugs. From cancer therapies to gene delivery systems, nanocarriers are providing more effective and precise treatments with fewer side effects. While there are still challenges related to safety, manufacturing, and regulatory approval, the potential benefits of nanotechnology in drug delivery are undeniable. As research continues and clinical trials progress, the future of nanotechnology in medicine looks incredibly bright.